Definition and Introduction: Transposition or transfer of cell, tissue or organ from one place to another or from one part of the same body to another part. The transfer may be between the same specie or between different species. The most transplanted tissue is blood other commonly transplanted tissues are the skin, bone, kidney, heart, and liver. The most transplanted solid organ is the kidneys.
Background/Historical perspective
The successful transplant of solid organs relies on 2 basic requirement
- Ability to restore blood supply to the transplanted tissue for its nourishment
- Ability to prevent rejection of the transplanted tissue by the immune system of the recipient
These two challenges were surmounted before transplant became more successful. The first requirement was met when methods of vascular anastomosis were described by Jaboulay and Alexis carrel and the second requirement when immunosuppressive properties of 6-mecaptopurine was uncovered by Schwartz and Dameshek (6-mecaptopurine discovery) and Calne demonstrated use of azathioprime which is a derivative of 6-mecaptopurine to prevent rejection of transplanted kidney in canine animal. .
The Landsteiner brothers discovered ABO blood grouping, and ABO compatibility is an absolute prerequisite before transplant
Terminologies
| Autograft | Self to self transplant |
| Allograft/Homograft | Between same specie but genetically dissimilar |
| Isograft/Syngenetic / Suggeneic | Same specie with similar genetic composition (identical twins) |
| Xenograft | Between different species (Discordant xenograft, there is preformed antibodies in host with risk of early rejection eg Pig to man, Concordant Xenograft; there is no preformed antibodies in host eg Between baboon and man with closer genetic similarities |
| Orthotropic site | Transplanted tissue placed where the original tissue normal embryologically eg heart and liver |
| Heterotropic site | Transplanted tissue placed at an ectopic site eg kidney |
| Privileged site | Site or tissue not vascularized hence does not require tissue typing or immunomodulation after transplant eg cornea transplant |
| Rejection | Identification of imported tissue as a foreign body followed by immunologic attempt to expel it. |
| HLA | Human Leucocyte Antigen. The expression of the antigen is by the Major Histocompatibility Complex(MHC). The genetic basis is on chromosome 6 |
Indication for transplantation: failure or loss of function of tissue/organ/ system. For example
failure of wound healing leading to chronic leg ulcer,
end stage renal failure,
cirrhosis and hepatic failure,
pulmonary failure eg cystic fibrosis ,
bone loss from tumor resection or traumatic fracture
Recipient selection
Consider age / life expectancy,
Establish the disease condition is non-recurrent,
Establish absence of severe co-morbidity that might interfere with transplant survival or maintenance eg HIV, HBV, immunosuppression -physical and psychological state of patient etc.
Donor selection
Exclude organ dysfunction
Exclude systemic disease or infections eg HIV, SCD
Same specie vs Different specie
Living VS cadaveric or brain dead donor.
Identicalènonidentical familyènon family HLA compatibleè non family HLA incompatible ( in order of decreasing preference)
Immunology of transplant
Immune response occurs when the host is exposed to antigen and antigen fragments from the transplanted tissue. The antigens inciting immune response and tissue rejection are the Histocompatibility Antigens.
Production of the HLA antigens :
The segment of genetic material coding for the histocompatibility antigens is the Major Histocompatibility complex (MHC) and the MHC is located on Chromosome 6. The MHC has 4 regions or Loci called HLA locus A, D, C & D.
The Histocompatibility Antigens are called Human Leucocyte Antigens (HLA) because they were first discovered in leucocytes. . The HLA are classified as HLA class I and class II.
Class I HLA
Coded for by MHC regions HLA A, B, C
Expressed on all nucleated cells
Targeted by Cytotoxic T-cells
Class II HLA
Coded for by MHC region HLA D ( HLA-DP, HLA-DQ and HLA-DR)
Expressed on Macrophages, Monocytes, B-lymphocytes and dendritic cells
Stimulates the Helper-T cells
Processing of the antigens and antigen fragments in circulation : The APC recognize and present the donor HLA to helper T cells Helper T Cells mediate activation of cytokines and cytotoxic T cells (cytotoxic T-cells). The Cytotoxic T cells induce transplant rejection the transplant.
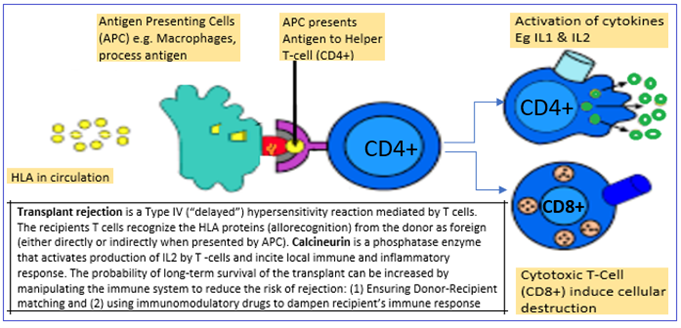
Problems of transplantation and the pathogenesis of the problems
Rejection types 🙁 Acute- humoral, Hyperacute-Cell mediated and Chronic- combination of humoral and cell mediated): incompatibility, antigen antibody reaction, vascular damage
Infection- immunosuppression
Graft versus host – immunosuppressed host, incompatibility
Pathogenesis of tissue rejection
Upon revascularization of transplanted tissue, antigen fragments from the transplanted tissue wash into circulation, the foreign antigens are identified by host immune cells , host produces antibodies attacking the vascular endothelium of transplanted tissue, inciting cytotoxicity, complement complex and coagulation with vascular thrombosis. The rejection process manifests as Vascular thrombosis,
loss of function, edema, and necrosis of the transplant
Rejection types (4 types ; hyperacute, accelerated, acute, chronic)
Hyperacute
Occurs within minutes of reperfusion of the transplant
Due to preformed antibodies in recipients circulation
The rejection type is not reversible but can be prevented
Due to antibodies directed at donor HLA or ABO-type antibodies
Accelerated acute : occurs few days posttransplant, mediated by humoral and CMI
Acute: Occurs within days and few months of transplant. Dominated by cell mediated immunity involving lymphocytes
Chronic
Occurs after months to years
Cannot be prevented , characterized by atrophy, fibrossys , arteriosclerosis with graft function deteriorating slowly
Management of problems of transplantation
Rejection- tissue typing, immune modulation
Infection- antibiotics reverse barrier nursing
Graft vs. host- tissue typing, immune modulation
Immunomodulation agents
| Anti Calcineurin Cyclosporine Tacrolimus (FK-506) similar to cyclosporine but 50 times more potent |
| mTOR inhibitors (mammalian Target of Rapamycin) inhititi IL2 receptor and T cell proliferation sirolimus, everolimus |
| Antimetabolites |
| Azathiaprime- depress DNA , RNA synthesis , depress lymphocyte proliferation Mycophenolate mofetil |
| Biological agents ( antilymphocyte globulins, monoclonal or polyclonal antibodies OKT3 monoclonal antibodies against helper T cells |
| Others Steroid : inhibit multiple pathways , inhibit lymphokine production Radiation |
Organ preservation /storage
In cadaveric donors, the interval between cardiac arrest and organ harvest is the warm ischemic time. The interval between harvesting and revascularization (transplant) is the cold ischemic time. After harvesting the metabolilc process can be slowed up to 12 fold by inducing hypothemia ( 40C) and using pharmacologic modalities to inhibit metabolism and reduce oxygen demands. After harvest, the organ is perfused with chilled preservation solution or stored in chilled organ can be stored in cold hyperosmotic , hyperkalemic solution such as University of winconsin solution and collins solution. The solution keeps the organ cold, prevent cells swelling and limit ion shifting. Different organ have different cold ischemic time
| Allowable cold ischemic time | |
| Kidney | 72 hours |
| Liver | 24 hours |
| Pancreas | 24 hours |
| Heart | 6 hours |
| Lung | 6 hours |
| Heart-lung | 6 hours |
| Bone | indefinitely |
| Skin | indefinitely |
| Cornea | indefinitely |
Ethical issues
Sales and commercialization of organs or tissue
Diagnosis of brain death
Conclusion:
Made replacement malfunctioning organ replaceable, technical, and expensive. Immune modulation and vascular anastomosis hold key to success